|
COVID-19 Breaking News
CDC Revises COVID-19 Vaccine Up to Date Definition
On May 24, the CDC released revised guidance documents changing the definition of “up to date” with COVID-19 vaccines, to include the recommendations for a second booster dose.
Key CDC Changes
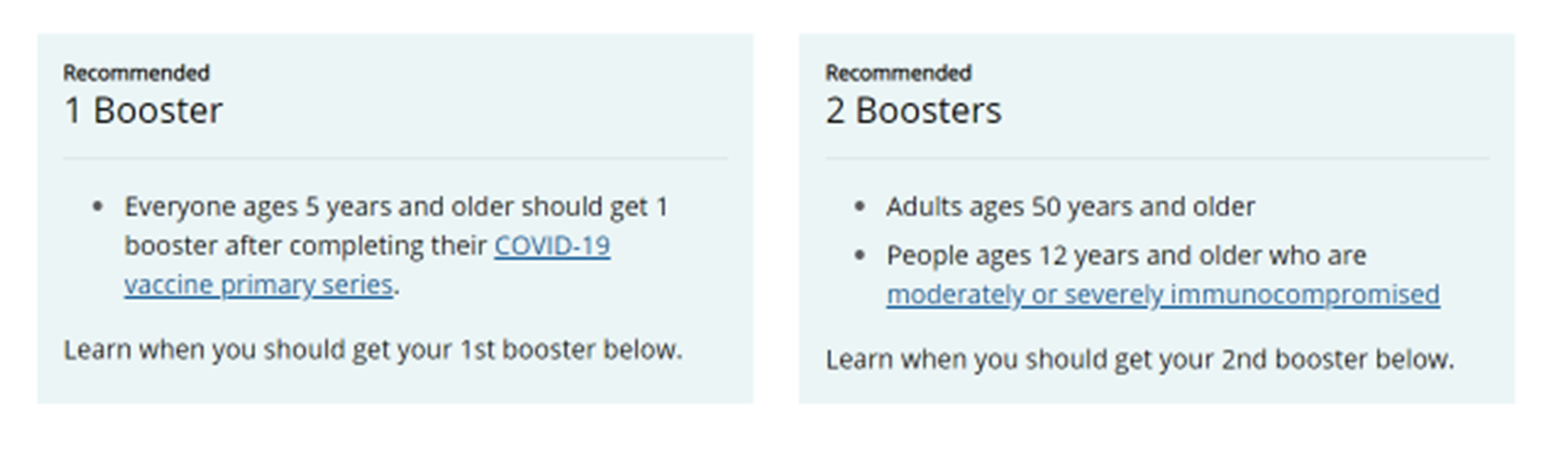
- For all individuals aged 50 and older, a second booster is now recommended once eligible AND included in the definition of up to date.
- For immunocompromised individuals aged 12 and older, a second booster is now recommended once eligible AND included in the definition of up to date.
Note: Ages 50+ and ages 12+ who are moderately or severely immunocompromised are eligible for a second booster 4 months after first booster.
What This Means?
The definition of up to date is utilized for:
The definition of up to date is not utilized for:
- Compliance with CMS Vaccine Mandate according to the QSO-22-09-All.
As a result of this change, LTC providers will need to establish a process of determining which staff members are eligible and recommended to receive a second booster dose. Based on the CDC’s recommendations contained in Stay Up to Date with Your COVID-19 Vaccines and the COVID-19 Booster Tool, this may be challenging because providers will need to track employee information with much greater detail including age and immunocompromised status.
Those staff who are now recommended to have the second booster once eligible (age 50+ and immunocompromised age 12+) but have not yet had it, must be removed from work if exposed, wear source control at all times regardless of community transmission rates, and participate in LTC surveillance testing. Note, this change does not affect CMS vaccine requirement compliance.
All residents (except someone under age 50) are now recommended to have the second booster once eligible. All residents who do not meet the new definition of up to date must be quarantined upon admission or readmission and remain in their rooms in the event of broad-based testing approaches in a COVID-19 outbreak.
LAI recommends adding additional columns to your COVID-19 Staff Vaccination Tracking to include whether the staff member is considered moderately or severely immunocompromised and adding either a date of birth or age to identify whether the staff member is recommended to have the second booster dose.
New CDC Recommendations for COVID-19 Vaccines (published May 24, 2022)
Individuals Not Considered Moderately or Severely Immunocompromised
Ages 50+:
- A primary series includes one of the following:
- Moderna. A two-dose series with the second dose being administered between 4-8* weeks following receipt of the first dose.
- Pfizer. A two-dose series with the second dose being administered between 3-8* weeks following receipt of the first dose.
- Johnson & Johnson’s Janssen*** which is a one-dose series.
- A first booster dose should be administered:
- Pfizer & Moderna. At least 5 months after the completion of the primary series for all individuals ages 5 and older.
- Johnson & Johnson’s Janssen. At least 2 months after the primary dose of a J&J vaccine.
- A second booster dose should be administered:
- Pfizer & Moderna. At least 4 months after the first booster dose.
Ages 18-49:
- A primary series includes one of the following:
- Moderna. A two-dose series with the second dose being administered between 4-8* weeks following receipt of the first dose.
- Pfizer. A two-dose series with the second dose being administered between 3-8* weeks following receipt of the first dose.
- Johnson & Johnson’s Janssen***. A one-dose series.
- A first booster dose should be administered:
- Pfizer & Moderna. At least 5 months after the completion of the primary series for all individuals ages 5 and older.
- Johnson & Johnson’s Janssen***. At least 2 months after the primary dose of a J&J vaccine.
Ages 5-17:
- A primary series of Pfizer. The first dose administered with a second dose administered at least 3-8* weeks after the first dose.
- A booster dose administered at least 5-months after completion of the primary series.
Moderately or Severely Immunocompromised Individuals
Ages 18+:
- A primary series includes one of the following:
- Moderna. A three-dose series with the second dose being administered 4** weeks following receipt of the first dose and a third dose being administered 4-weeks following receipt of the second dose.
- Pfizer. A three-dose series with the second dose being administered 3** weeks following receipt of the first dose and a third dose being administered 4-weeks following receipt of the second dose.
- Johnson & Johnson’s Janssen***. A two-dose series with the second dose being administered at least 4-weeks (28 days) after the first dose.
- A first booster dose should be administered:
- Pfizer & Moderna. At least 3 months after the completion of the primary series.
- Johnson & Johnson’s Janssen***. At least 2 months after the primary series of a J&J vaccine.
- A second booster dose should be administered:
- Pfizer & Moderna. At least 4 months after the fourth dose (or first booster dose). Note individuals who received the J&J vaccine as a primary and/or booster vaccine should receive an mRNA vaccine for the second booster dose. J&J cannot be used as a second booster dose.
Ages 12-17:
- A primary series of Pfizer. A 3-dose primary series including a second dose administered 3 weeks (21 days) after the first dose, and a third dose administered 4 weeks (28 days) after the second dose.
- A Pfizer booster dose administered at least 3-months after the final dose in the primary series.
- A Pfizer 2nd booster dose administered at least 4-months after the first booster.
Ages 5-11:
- A primary series of Pfizer. A 3-dose primary series including a second dose administered 3 weeks (21 days) after the first dose, and a third dose administered 4 weeks (28 days) after the second dose.
- A Pfizer booster dose administered at least 3-months after the final dose in the primary series.
*People ages 5 through 64 years, and especially males ages 12 through 39 years, may consider getting the second primary dose of an mRNA COVID-19 vaccine 8 weeks after the first dose. A longer time between the first and second primary doses may increase how much protection the vaccines offer, and further minimize the rare risk of heart problems, including myocarditis and pericarditis.
**Peoples ages 65 years and older, people more likely to get very sick from COVID-19, or anyone wanting protection due to high levels of community transmission should get the second primary dose of Pfizer’s COVID-19 vaccine 3 weeks (or 21 days) after the first primary dose, or the second primary dose of Moderna’s COVID-19 vaccine 4 weeks (or 28 days) after the first primary dose.
***Refer to CDC’s Johnson & Johnson’s Janssen COVID-19 Vaccine: Overview and Safety for when the J&J vaccine is appropriate for use.
|