General
The CDC notes that the revised guidance for healthcare personnel reflects high levels of vaccine and infection-induced immunity and the availability of effective treatments and prevention tools. The guidance provides a framework for healthcare providers to implement select infection prevention and control practices based on individual circumstances. The revised guidance is intended for all healthcare providers (including nursing homes and home health); there is no longer a separate set of guidance for nursing homes.
Determining COVID-19 Transmission:
All healthcare providers should continue to use the Community Transmission metric on the CDC COVID-19 Data Tracker.
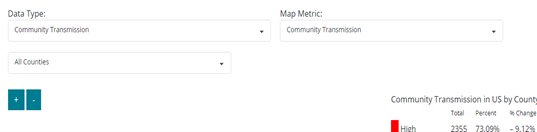
Do NOT use the Community Levels on the CDC COVID-19 Data Tracker.
Recommended Routine IPC Practices:
- Encourage everyone to remain up to date with all recommended COVID-19 vaccine doses.
- Post visual alerts at the entrance and in strategic places throughout the building. These visual alerts should include instructions about current IPC recommendations such as performing hand hygiene and when to use source control.
According to the CMS QSO-20-39-NH the Core Principles of COVID-19 Infection Prevention include:
- Nursing homes should provide guidance (such as posted signs at entrances) about recommended actions for visitors who have a positive viral test for COVID-19, symptoms of COVID-19, or have had close contact with someone with COVID-19. Visitors with confirmed COVID-19 infection or compatible symptoms should defer non-urgent in-person visitation until they meet CDC criteria for healthcare settings to end isolation. For visitors who have had close contact with someone with COVID-19 infection, it is safest to defer non-urgent in-person visitation until 10 days after their close contact if they meet criteria described in CDC healthcare guidance (such as not wearing source control).
- Hand Hygiene (use of alcohol-based hand rub is preferred).
- Face covering or mask (covering mouth and nose) in accordance with CDC guidance.
- Instructional signage through the building and proper visitor education on COVID-19 signs and symptoms, infection control precautions, other applicable nursing home practices (such as use of face covering or mask, specified entries, exits and routes to designated areas, hand hygiene).
- Cleaning and disinfecting high-frequency touched surfaces in the nursing home often, and designated visitation areas after each visit.
- Appropriate staff use of PPE.
- Effective cohorting of residents.
- Resident and staff testing conducted as required.
Screening
Healthcare providers should establish a process to make everyone entering the building aware of recommended actions to prevention transmission to others if they have any of the following criteria:
It is no longer required that healthcare providers have a person screening each individual that enters the building. However, providers must ensure that they have a process that educates all individuals on the guidance and recommended actions for anyone meeting the above criteria. For example:
- HCP could be instructed to report to occupational health or another designated point of contact so they can be properly managed.
- Posted signs at entrances for guidelines regarding patients and visitors who have any of the above criteria.
- Visitors with confirmed COVID-19 infection or compatible symptoms should defer non-urgent in-person visitation until they meet healthcare criteria to end isolation, which is longer than what is recommended in the community. For visitors who had close contact with someone confirmed with COVID-19 infection or were in another situation that puts them at a higher-risk for transmission, it is safest to defer non-urgent in-person visitation until 10 days after their close contact if they cannot adhere to IPC recommendations (such as wearing a mask).
It is no longer required to screen residents on a daily basis for symptoms of COVID-19 or increasing the frequency of this screening during an outbreak.
Source Control and PPE
Visitors: Healthcare providers may choose to offer well-fitting facemasks as source control options for visitors but should allow the use of a mask or respirator with higher-level protection that is not visibly soiled by individuals that choose that option based on their individual preference.
Source control options for HCP include:
- A NIOSH-approved particulate respirator with N95 filters or higher.
- A respirator approved under standards used in other countries that are similar to NIOSH-approved N95 filtering facepiece respirators (Note these should not be used instead of a NIOSH-approved respirator when respiratory protection is indicated.)
- A barrier face covering that meets ASTM F3502-21 requirements including Workplace Performance and Workplace Performance Plus masks, (note the link in the CDC document to the ASTM F3502-21 standards is not active) or
- A well-fitting facemask.
The above source control options can be utilized for an entire shift, unless they become soiled, damaged, or hard to breathe through. When used during a surgical procedure or during care of a patient on droplet precautions the source control should be removed and discarded with a new one donned.
When to Wear Source Control:
When Community Transmission levels are high, source control is recommended for everyone in a healthcare setting when they are in areas of the building where they could encounter patients.
When Community Transmission levels are not high, healthcare providers could choose not to require universal source control. However, even if source control is not universally required, it remains recommended for individuals who:
- Have suspected or confirmed COVID-19 infection or other respiratory infection (such as those with a runny nose, cough, or sneezing); or
- Has close contact or a higher-risk exposure with someone with COVID-19 infection for 10 days after their exposure; or
- Reside or work on a unit or area of the building experiencing a COVID-19 outbreak (note outbreak is still defined as 1 positive case). Universal source control could be discontinued as a mitigation measure once no new cases have been identified for 14-days; or
- Have otherwise had source control recommended by public health authorities.
Individuals might also choose to continue wearing source control based on personal preference, informed by their perceived level of risk for infection based on their recent activities (such as attending crowded indoor gatherings with poor ventilation) and their potential for developing severe disease. Providers and HCP may also consider using or recommending source control when caring for patients who are moderately to severely immunocompromised.
Use of PPE for HCP:
If COVID-19 infection is not suspected in a patient presenting for care, HCP should follow standard precautions. If a patient is suspected to have a diagnosis that would require use of transmission-based precautions, HCP should implement PPE based on the suspected diagnosis.
As community transmission levels increase, the potential for encountering asymptomatic or pre-symptomatic individuals with COVID-19 likely increases. In these circumstances, providers should consider implementing broader use of respirators and eye protection by HCP during patient care encounters. For example, providers located in counties where community transmission is high should also consider having HCP use PPE including:
- A NIOSH-approved particulate respirator with N95 filters or higher for:
- All aerosol-generating procedures.
- HCP working in situations where additional risk factors for transmission are present, such as when the patient is unable to use source control and the area is poorly ventilated.
- If health-care associated transmission is identified and universal respirator use is not already in place.
- To simply implementation, providers in counties with high transmission may consider implementing universal use of a NIOSH-approved respirator with N95 filers or higher for all patient care encounters or in specific units of the building at higher risk for COVID-19 transmission.
- Eye protection (such as goggles or a face shield that covers the front and sides of the face) during all patient care encounters.
Optimizing the Use of Engineering Controls and Indoor Air Quality
Providers should optimize the use of engineering controls to reduce or eliminate exposures by shielding HCP and other patients from infected individuals which could include physical barriers at reception/triage locations and dedicated pathways to guide symptomatic patients through triage areas.
Take measures to limit crowding in communal spaces, such as limiting the numbers of patients in waiting rooms or treatment areas.
Explore options, in consultation with engineers, to improve ventilation delivery and indoor air quality in patient rooms and shared spaces. Guidance on ensuring that ventilation systems are operating properly, and other options for improving indoor air quality are available at the following resources:
COVID-19 Testing
The revised CMS QSO-20-38-NH provides the following interpretive guidance for COVID-19 testing regulations.
For CMS testing requirements, staff is defined as employees, consultants, contractors, volunteers, and caregivers who provide care and services to residents on behalf of the nursing home, and students in the nurse aide training programs or from affiliated academic institutions.
|
Testing Trigger
|
Staff
|
Residents
|
|
Symptomatic individual identified
|
Staff, regardless of vaccination status, with signs or symptoms must be tested as soon as possible.
|
Residents, regardless of vaccination status, with signs or symptoms must be tested as soon as possible.
|
|
Newly identified COVID-19 positive staff or resident in a nursing home that can identify close contacts
(Outbreak and Exposure Testing)
|
Test all asymptomatic staff, regardless of vaccination status, that had a higher-risk exposure with a COVID-19 positive individual.
Exposure testing should occur immediately (but not sooner than 24 hours) with two additional tests, at least 48 hours after the previous test.
For example: Exposure day is day 0, test on day 1, 3, and 5.
|
Test all asymptomatic residents, regardless of vaccination status, that had close contact with a COVID-19 positive individual.
Exposure testing should occur immediately (but not sooner than 24 hours) with two additional tests, at least 48 hours after the previous test.
For example: Exposure day is day 0, test on day 1, 3, and 5.
|
|
Newly identified COVID-19 positive staff or resident in a nursing that is unable to identify close contacts
(Outbreak broad-based testing)
|
Test all staff, regardless of vaccination status, facility-wide or at a group level if staff are assigned to a specific location where the new case occurred (unit, floor, or other specific area(s) of the nursing home.
Testing should occur every 3-7 days until no additional cases are identified for a period of 14 days.
|
Test all residents, regardless of vaccination status, facility-wide or at a group level (unit, floor, or other specific area(s) of the nursing home.
Testing should occur every 3-7 days until no additional cases are identified for a period of 14 days.
|
|
Routine Testing (Also referred to as screening or surveillance testing)
|
Not generally recommended.
|
Not generally recommended.
|
CDC and CMS revised the criteria for testing individuals who have recently recovered from COVID-19. The previous guidance indicated that individuals do not need to be tested if they’ve recovered from COVID-19 in the last 90 days. The new guidance states:
- Due to challenges interpreting the result, testing is generally not recommended for asymptomatic people who have recovered from COVID-19 within in the last 30 days. Testing should be considered for those who have recovered in the prior 31-90 days; however, an antigen test instead of a NAAT is recommended.
Resident Testing on Admission:
Screening testing in patients is likely lower for identifying asymptomatic infection, particularly in lower levels of community transmission. If providers choose to implement a screening testing program, decisions should not be based on vaccination status of the individual. If using antigen testing instead of NAAT, providers should use a series of 3 tests, spaced 48 hours apart, in line with FDA recommendations.
- In nursing homes, new admissions and residents who leave the building for more than 24 hours, when the county transmission level is high, should be tested with a series of 3 tests, spaced 48 hours apart.
- In nursing homes, new admissions and residents who leave the building for more than 24 hours, when the county transmission level is not high, is at the discretion of the provider.
Resident Refusal of Testing:
Residents may exercise their right to decline COVID-19 testing. In discussing testing with residents, staff should use person-centered approaches when explaining the importance of testing for COVID-19. Providers must have procedures in place to address residents who refuse testing. Procedures should ensure that residents who refuse testing are managed in accordance with CDC’s guidance for transmission-based precautions.
HCP Testing:
Generally, routine testing of HCP based on community transmission levels is not recommended, regardless of vaccination status.
Outbreak Testing:
If healthcare-associated transmission is suspected or identified, providers might consider expanded testing of HCP and patients as determined by the distribution of cases throughout the building. For example, providers may elect to initially expand testing only to HCP and patients on affected units or departments, or particular schedule or shift as opposed to the entire building. If expanded testing approaches are taken, and new cases are identified, testing should be expanded more broadly and if possible, repeated every 3-7 days until no new cases are identified for at least 14-days.
- Providers responding to healthcare-associated transmission should always notify and follow the recommendations of public health authorities.
IPC Practices for Patients with Symptoms or Close Contact
Empiric Transmission Based Precautions (TBP) for Symptomatic Patients:
Providers should place symptomatic individuals in TBP until:
- A single NAAT test indicates a negative result. If a higher level of clinical suspicion for COVID-19 infection exists, consider maintaining TBP and confirming with a second NAAT.
- If using an antigen test, a negative test should be confirmed by either a negative NAAT test, or a second antigen test collected 48 hours after the first.
- If testing is not conducted, the discontinuation of TBP can be based on time from symptom onset as described in the isolation section. Ultimately, clinical judgement and suspicion of COVID-19 infection determines whether to continue or discontinue empiric TBP. (LAI recommends clearly documenting clinical judgement reasoning for individuals who have not met the discontinuation of isolation criteria.)
Empiric TBP for Patients with Close-Contact:
Generally, asymptomatic patients do not require empiric use of TBP (regardless of vaccination status). These patients should wear source control and those who have not recovered from COVID-19 within the last 30-days should be tested on day 1, 3, and 5 as outlined in the testing section.
Providers should consider empiric TBP following close contact when:
- The patient is unable to be tested or wear source control as recommended for the 10-days following exposure.
- The patient is moderately to severely immunocompromised.
- The patient is residing on a unit with others who are moderately to severely immunocompromised.
- The patient is residing on a unit with ongoing COVID-19 transmission that is not controlled with initial interventions.
Patients discretionarily placed in empiric TBP based on close contact can be removed:
- After day 7 following the exposure (day of exposure is day 0), if they do not develop symptoms and all viral testing is negative.
- If testing is not performed, patients can be removed from TBP after day 10 following the exposure (day of exposure is day 0) if they do not develop symptoms.
IPC Practices for Patients with Suspected or Confirmed COVID-19 Infection
Patient Placement for Patients with Suspected or Confirmed COVID-19 Infection:
Patients suspected or confirmed with COVID-19 should ideally be placed in a single-person room with the door kept shut (if safe to do so) with a dedicated bathroom.
- If cohorting, only patients with the same respiratory pathogen should share rooms. MDRO colonization status and/or presence of other communicable diseases should also be taken into consideration during the cohorting process.
Providers should consider designating entire units with dedicated HCP to care for patients with COVID-19 infection when the number of patients with COVID-19 infection is high. Dedicated units and/or HCP might not be feasible due to staffing crises or a small number of patients with COVID-19 infections.
- In nursing homes, if limited single rooms are available, or if numerous residents are simultaneously identified to have known COVID-19 exposure or symptoms, should remain in their current location.
Limit transport and movement of the patient outside of the room to medically essential purposes.
Communicate information about COVID-19 status to appropriate personnel before transferring them to other departments and/or other healthcare providers.
Duration of Patient TBP for Confirmed COVID-19 Illness:
Note these guidelines have not changed.
Patients with mild to moderate illness who are not moderately to severely immunocompromised:
- At least 10 days have passed since symptoms first appeared; and
- At least 24-hours have passed since last fever without the use of fever-reducing medications; and
- Symptoms have improved.
Patients who were asymptomatic throughout their infection and are not moderately to severely immunocompromised:
- At least 10 days have passed since the date of their first positive test.
Patients with severe to critical illness and who are not moderately to severely immunocompromised:
- At least 10 days and up to 20 days have passed since symptoms first appeared and
- At least 24 hours have passed since last fever without the use of fever-reducing medications and
- Symptoms have improved
- The test-based strategy for moderately to severely immunocompromised patients can be used to inform the duration of isolation.
Patients who are moderately to severely immunocompromised:
Patients who are moderately to severely immunocompromised may produce replication-competent virus beyond 20 days after symptom onset or, for those who were asymptomatic throughout their infection, the date of their first positive viral test. Use of a test-based strategy and (if available) consultation with an infectious disease specialist is recommended to determine when TBP could be discontinued.
The criteria for symptomatic patients utilizing a test-based strategy include:
- Resolution of fever without the use of fever-reducing medications and
- Symptoms have improved, and
- Results are negative from at least two consecutive respiratory specimens collected 48 hours apart (total of two) using an antigen or NAAT.
The criteria for asymptomatic patients utilizing a test-based strategy include two consecutive respiratory specimens collected 48 hours apart (total of two) using an NAAT or antigen test.
HCP PPE for Patients with Suspected or Confirmed COVID-19 Infection:
HCP who enter the room of a patient with suspected or confirmed COVID-19 should adhere to TBP including the use of a NIOSH-approved particulate respirator with N95 filters or higher, gown, gloves, and eye protection.
- Respirators should be used in the context of a comprehensive respiratory protection program, which includes medical evaluations, fit testing, and training in accordance with the Occupational Safety and Health Administration’s Respiratory Protection Standards (29 CFR 1910.134). LeadingAge Iowa’s OSHA Respiratory Protection Program template
- Additional information about using PPE is available in Protecting Healthcare Personnel.
Aerosol-Generating Procedures (AGPs):
- Procedures that could generate infectious aerosols should be performed cautiously and avoided if appropriate alternatives exist.
- AGPs should take the place in airborne infection isolation rooms (AIIR), if possible.
- The number of HCP present during aerosol generating procedures should be limited to only those essential for patient care and procedure support. Visitors should not be present for the procedure.
Environmental Infection Control:
- Dedicated medical equipment should be used when caring for patients with suspected or confirmed COVID-19. Any non-dedicated, non-disposable medical equipment used should be cleaned and disinfected according to manufacturer’s instructions and policy and procedures before use on another patient.
- Routine cleaning and disinfection procedures according to the EPA List N that kill COVID-19 should be selected and appropriate for other pathogens of concern including C. difficile.
- Management of laundry, food service utensils, and medical waste should be performed in accordance with policies and procedures for a patient in TBP.
- Once the patient has been discharged or transferred, HCP should refrain from entering the vacated room without all recommended PPE until sufficient time has elapsed for enough air changes to remove potentially infectious particles (see guidance on clearance rates under differing ventilation conditions). After this time has elapsed, the room should undergo appropriate cleaning and surface disinfection before it is returned to routine use.
Visitation When a Patient is in Isolation:
For the safety of the visitor, in general, patients should be encouraged to limit in-person visitation while they are infectious. However, providers should adhere to regulations related to visitation. If visitation occurs:
- Counsel patients and visitor(s) about the risks of an in-person visit.
- Encourage use of alternate mechanisms for patient and visitor interactions such as video-call applications on cell phones or tablets when appropriate.
- Providers should include instruction, before visitors enter the room on hand hygiene, limiting surfaces touched, and the use of PPE to current provider policy.
- Visitor(s) should be instructed to only visit the patient room and minimize time spent in other locations in the building.
ICP for HCP with Higher-Risk Exposures
HCP are now allowed to work, regardless of vaccination status, following a known higher-risk exposure as long as the following can be met:
- Have a series of 3 viral tests for COVID-19:
- Testing is recommended immediately (but not earlier than 24 hours after the exposure) and, if negative, an additional 2-tests 48 hours apart. Ideally this is day 1 (where exposure is day 0), day 3 and day 5.
- HCP who have recovered from COVID-19 infection in the prior 30 days, are not recommended for testing as long as they are asymptomatic. COVID-19 testing should be considered for those who have recovered from COVID-19 in the past 31-90 days, however, an antigen test instead of a NAAT is recommended.
- Follow all recommended infection prevention and control practices, including wearing well-fitting source control, monitoring themselves for fever or symptoms consistent with COVID-19 and not reporting to work when ill or if testing positive for COVID-19.
- Any HCP who develops a fever or symptoms consistent with COVID-19 should immediately self-isolate and contact their established point of contact at the provider to arrange for medical evaluation and testing.
The provider may determine that work restriction is necessary. Examples of when work restriction may be considered:
- HCP is unable to be tested or wear source control as recommended for the 10 days following their exposure.
- HCP is moderately to severely immunocompromised.
- HCP cares for or works on a unit with patients who are moderately to severely immunocompromised.
- HCP works on a unit experiencing ongoing COVID-19 transmission that is not controlled with initial interventions.
If work restriction is recommended, the HCP can return to work after either of the following time periods:
- HCP can return to work after day 7 following the exposure (day 0) if they do not develop symptoms and all viral testing as described for asymptomatic HCP following a higher-risk exposure is negative.
- If viral testing is not performed, HCP can return to work after day 10 following the exposure (day 0) if they do not develop symptoms.
Employee Return to Work after COVID-19 Infection
The employee return to work criteria following COVID-19 infection has not changed.
CDC also revised the document Strategies to Mitigate Healthcare Personnel Staffing Shortages, reflecting that HCP are not required to quarantine following a high-risk exposure, regardless of their vaccination status under conventional staffing strategies.
The contingency and crisis staffing strategies have not changed. As a reminder, contingency and crisis staffing strategies must be implemented sequentially and documented when they are implemented.
Setting Specific Considerations
Nursing Homes:
IPC Program Management: Nursing home providers should assign one or more individuals with training in IPC to provide on-site management of the IPC program. CDC recommends at least a full-time role for providers that have 100 or more residents or that provide on-site ventilator or hemodialysis services.
NHSN Reporting: Report COVID-19 data to the NHSN LTCF COVID-19 Module according to CMS reporting requirements.
Visitation: CMS revised QSO-20-39-NH with the following changes (in addition to those noted in other sections like COVID-19 outbreaks of this article):
- During peak times of visitation and large gatherings (such as parties, events) nursing home should encourage physical distancing.
- If the nursing home’s county COVID-19 community transmission is high, everyone in a healthcare setting should wear face coverings or masks.
- If the nursing home’s county COVID-19 community transmission rate is not high, the safest practice is for residents and visitors to wear face coverings or masks, however, the nursing home could choose not to require visitors to wear face coverings or masks while in the building (except during an outbreak). The nursing home’s policies regarding face coverings should be based on recommendations from the CDC, state and local public health, and individual nursing home circumstances.
- Regardless of the community transmission level, residents and their visitors, when along in the resident’s room or designated visiting area, may choose not to wear face coverings or masks and may choose to have close contact (including touch). Residents (or their representatives) and their visitors should be advised of the risks of physical contact prior to the visit.
- If a roommate is present during the visit, it is safest for the visitor to wear a face covering or mask.
COVID-19 Outbreaks:
- Outbreak response by nursing homes should follow recommendations of the local public health authorities.
- A single new case of COVID-19 infection in any HCP or resident should be evaluated to determine if others in the building could have been exposed.
- Outbreak investigation could involve either contact tracing or broad-based approaches. A broad-based approach is preferred if all potential contacts cannot be identified or managed with contact tracing or if contact tracing fails to halt transmission. Testing based strategies for contact tracing and broad based approaches are noted in the testing section.
- Visitation during outbreak response:
- CMS noted (in addition to previous recommendations) in QSO-20-39-NH that during an outbreak investigation, nursing homes should limit visitor movement in the building. For example, visitors should not walk around different halls of the building. Visitors should also physically distance themselves from other residents and staff, when possible.
- Visitors should be counseled about their potential to be exposed to COVID-19.
- If indoor visitation is occurring in areas of nursing home transmission, it should ideally occur in the resident’s room. The resident and their visitors should wear well-fitting source control (if tolerated) and physically distance (if possible) during the visit.
CMS noted in QSO-20-38-NH that residents who are admitted directly into TBP (based on provider’s discretion) and develops COVID-19 prior to TBP being discontinued should not trigger an outbreak investigation for the nursing home.
In QSO-20-39-NH, CMS also noted in the FAQ section, that communal activities and dining do not have to be paused during an outbreak, unless directed by the state or local public health department. Residents who are on TBP (isolation or quarantine) should not participate in communal activities and dining until the criteria to discontinue TBP has been met.
Nursing Home Admissions and Residents That Leave the Building for more than 24 hours (note this is no longer based on residents vaccination status):
- Admissions in counties where community transmission levels are high should be tested upon admission. Testing is recommended at admission with a repeat test 48 hours after the first negative result.
- Residents should also be advised to wear source control for the 10 days following their admission.
- Residents should be advised to wear source control for the 10 days following their admission.
- Residents who leave the building for 24 hours or longer should generally be managed as an admission.
- Empiric use of TBP is generally not necessary for admissions or for residents who leave the building for less than 24 hours and do not meet criteria in the suspected or confirmed COVID-19 infection section.
Assisted Living, Group Homes and Other Residential Care Settings (Excluding Nursing Homes):
In general, long-term care settings whose staff provide non-skilled personal care similar to that provided by family members in the home, should follow community prevention strategies based on COVID-19 community levels, similar to independent living, retirement communities or other non-healthcare congregate settings.
Tenants should also be counseled about strategies to protect themselves and others, including recommendations for source control if they are immunocompromised or at high risk for severe disease. CDC has information and resources for older adults and for people with disabilities.
Visiting or shared healthcare personnel who enter the setting to provide healthcare to one or more residents (such as physical therapy, wound care, home health agency nurses) should follow the healthcare IPC recommendations in this guidance. In addition, if staff in a residential care setting are providing in-person services for a resident with COVID-19 infection, they should be familiar with recommended IPC practices to protect themselves and others from potential exposures including hand hygiene, PPE, and cleaning and disinfection practices outlined in this guidance.
Non-skilled personal care consists of any non-medical care that can reasonably and safely be provided by non-licensed caregivers, such as help with daily activities like bathing and dressing. It may also include the kind of health-related care that most people do themselves, like taking oral medications. In some cases where care is received at home or a residential setting, care can also include help with household duties such as cooking and laundry.
LAI Resources:
References
It’s important to note that the CDC archived the document “Interim Infection Prevention and Control Recommendations to Prevent SARS-CoV-2 Spread in Nursing Homes” and incorporated the information into the Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the COVID-19 Pandemic document. This article incorporates the following guidance documents:
CDC Revises Mitigating Staff Shortages Guidance
On September 23, the CDC released a revised guidance document on Strategies to Mitigating Staffing Shortages. The CDC noted that HCP with a higher-risk exposure to COVID-19 may work, regardless of vaccination status under conventional strategies, however, additional strategies were added to Contingency plans. As a reminder, these measures are meant to implemented sequentially, with documentation of when each strategy has been implemented. LeadingAge Iowa is working on revising the Mitigating Staffing Strategies Flowchart and will post to the website when available.
The CDC also noted that allowing HCP infected with COVID-19 to return to work before meeting the conventional criteria could result in healthcare-associated transmission. Providers (in collaboration with risk management) should inform patients and other HCP when these strategies are being utilized, specify the changes in practice that should be expected, and describe the actions that will be taken to protect patients and HCP from exposure to COVID-19 if HCP with suspected or confirmed COVID-19 infection are requested to work to fulfill staffing needs.
Conventional Strategies:
- Ensure any COVID-19 vaccine requirements for HCP are followed, and where none are applicable, encourage HCP to remain up to date with all recommended COVID-19 vaccine doses.
- Understand normal staffing needs and the minimum number of staff needed to provide a safe work environment and safe patient care under normal circumstances.
- Understand the local epidemiology of COVID-19 related indicators (such as community transmission levels).
- Communicate with local healthcare coalitions and federal, state, and local public health partners (such as public health emergency preparedness and response staff) to identify additional HCP (such as hiring additional HCP, recruiting retired HCP, using students or volunteers), when needed.
Contingency Capacity Strategies:
When staffing shortages are anticipated, healthcare facilities and employers, in collaboration with human resources and occupational health services, should use contingency capacity strategies to plan and prepare for mitigating this problem.
- Adjusting staff schedules, hiring additional HCP, and rotating HCP to positions that support patient care activities.
- Cancelling all non-essential procedures and visits. Shift HCP who work in these areas to support other patient care activities in the building. Providers will need to ensure these HCP have received appropriate orientation and training to work in these areas that are new to them.
- Attempt to address social factors that might prevent HCP from reporting to work, such as need for transportation or housing that allows for physical distancing, particularly if HCP live with individuals with underlying medical conditions or older adults.
- Consider that these social factors disproportionately affect persons from some racial and ethnic groups, who are also disproportionally affected by COVID-19.
- Identify additional HCP to work in the building. Be aware of state-specific emergency waivers or changes to licensure requirements or renewals for select categories of HCP.
- As appropriate, request that HCP postpone elective time off from work. However, there should be consideration for the mental health benefits of time off and that care-taking responsibilities may differ substantially among staff.
- Developing regional plans to identify designated healthcare facilities or alternate care sites with adequate staffing to care for patients with COVID-19 infection.
- Allowing HCP with COVID-19 infection who are well enough and willing to work to return to work as follows:
- HCP with mild to moderate illness who are not moderately to severely immunocompromised:
- At least 5 days have passed since symptoms first appeared (day 0), and
- At least 24 hours have passed since last fever without the use of fever-reducing medications, and
- Symptoms have improved.
- HCP who were asymptomatic throughout their infection and are not moderately to severely immunocompromised:
- At least 5 days have passed since the date of their first positive viral test (day 0).
- Providers could consider to confirm resolution of infection with a negative NAAT or a series of negative antigen tests taken 48 hours apart.
- Considerations for determining which HCP should be prioritized for this option:
- The type of HCP shortages that need addressed.
- The types of symptoms they are experiencing.
- Their degree of interaction with patients and other HCP in the building.
- The type of patients they care for.
- If HCP are requested to return to work before meeting all conventional return to work criteria should still adhere to recommendations:
- They should self-monitor symptoms and seek re-evaluation from occupational health if symptoms recur or worsen.
- Until they meet the conventional return to work criteria:
- They should wear a respirator or well-fitting facemask at all times, even when they are in non-patient care areas such as breakrooms.
- If they must remove their respirator or well-fitting facemask, for example, in order to eat or drink, they should separate themselves from others.
- To the extent possible they should practice physical distancing from others.
- Patients (if tolerated) should wear well-fitting source control while interacting with these HCP.
Crisis Capacity Strategies to Mitigate Staffing Shortages:
- Implement regional plans to transfer patients with COVID-19 to designated healthcare providers, or alternate care sites with adequate staffing.
- If shortages continue despite other mitigation strategies, as a last resort consider allowing HCP to work even if they have suspected or confirmed COVID-19 infection, if they are well enough and willing to work, even if they have not met all contingency return to work criteria described above.
- Considerations for determining which HCP should be prioritized for this option:
- The type of HCP shortages that need addressed.
- The types of symptoms they are experiencing.
- Their degree of interaction with patients and other HCP in the building.
- The type of patients they care for.
- If HCP are requested to work before meeting all criteria, they should be restricted from contact with patients who are moderately to severely immunocompromised and providers should consider prioritizing their duties in the following order:
- If not already done, allow HCP with suspected or confirmed COVID-19 to perform job duties where they do not interact with others, such as telemedicine.
- Allow HCP with confirmed COVID-19 to provide direct care only to patients with confirmed COVID-19 infection, preferably in a cohort setting.
- Allow HCP with confirmed COVID-19 infection to provide direct care only for patients with suspected COVID-19 infection.
- As a last resort, allow HCP with confirmed COVID-19 infection to provide direct care for patients without suspected or confirmed COVID-19 infection. If this is being considered, this should be used only as a bridge to longer term strategies that do not involve care of uninfected patients by potentially infectious HCP. Strict adherence to all other recommended infection prevention and control measures is essential.
- If HCP are requested to return to work before meeting all conventional return to work criteria should still adhere to recommendations:
- They should self-monitor symptoms and seek re-evaluation from occupational health if symptoms recur or worsen.
- Until they meet the conventional return to work criteria:
- They should wear a respirator or well-fitting facemask at all times, even when they are in non-patient care areas such as breakrooms.
- If they must remove their respirator or well-fitting facemask, for example, in order to eat or drink, they should separate themselves from others.
- To the extent possible they should practice physical distancing from others.
- Patients (if tolerated) should wear well-fitting source control while interacting with these HCP.















